Brenna Jenny
Brace yourselves for stricter healthcare and life sciences industry regulations and enforcement under President Joe Biden, and a Democratic-controlled Senate. Particularly in mergers and acquisitions; antitrust laws; drug pricing; and telehealth.
On Feb. 1, Sidley Austin LLP said Brenna Jenny rejoined the firm’s global healthcare and FDA practice. She was Principal Deputy General Counsel for the U.S. Department of Health and Human Services (HHS) and the Chief Legal Officer for the Centers for Medicare & Medicaid Services (CMS). In her prior roles, Jenny oversaw numerous CMS adjustments made following the COVID-19 pandemic. Working in the Department of Justice’s Civil Division, Jenny served as legal counsel, supervising False Claims Act matters and other healthcare-related lawsuits.
In a departure from the Trump administration, Sidley’s Jenny, Jaime Jones, Paul Kalb and Wandaly Fernandez explain how the Department of Justice (DOJ) will be more active in enforcement. As it relates to healthcare consumer protection, they write that the HHS and DOJ’s antitrust division will likely address more anticompetitive practices in the healthcare industry.
The appointment of Xavier Becerra as Secretary of Health and Human Services complements this antitrust sentiment. Serving as California Attorney General, Becerra is known for targeting healthcare and life science industries on antitrust matters. In December 2019, Becerra made headlines with his $575 million antitrust settlement with Sutter Health, effectively shutting down its alleged anticompetitive practices. His nomination would make the HHS an active participant in regulating issues like drug pricing, marketing practices, and healthcare consolidation.
Furthermore, Sen. Amy Klobuchar also proposed stricter antitrust enforcement. Now chair of the Subcommittee on Antitrust, Klobuchar’s proposed bill would provide additional funding for the Federal Trade Commission. The bill would improve consumer protection enforcement regarding both Big Tech and life science industries. Under Biden, greater antitrust enforcement would address the consolidation of healthcare services that have contributed to the increases in healthcare costs.
Like that of the Trump administration, the HHS under Biden will continue to pursue lowering drug pricing. Sidley opines:
HHS actions will target the following areas: price transparency, price rate limits on specialized drugs, limits on drug price increases, and promotion of quality generic drugs.
In doing so, FCA suits are likely to be raised against high drug prices and other patient support programs that undermine drug price regulations.
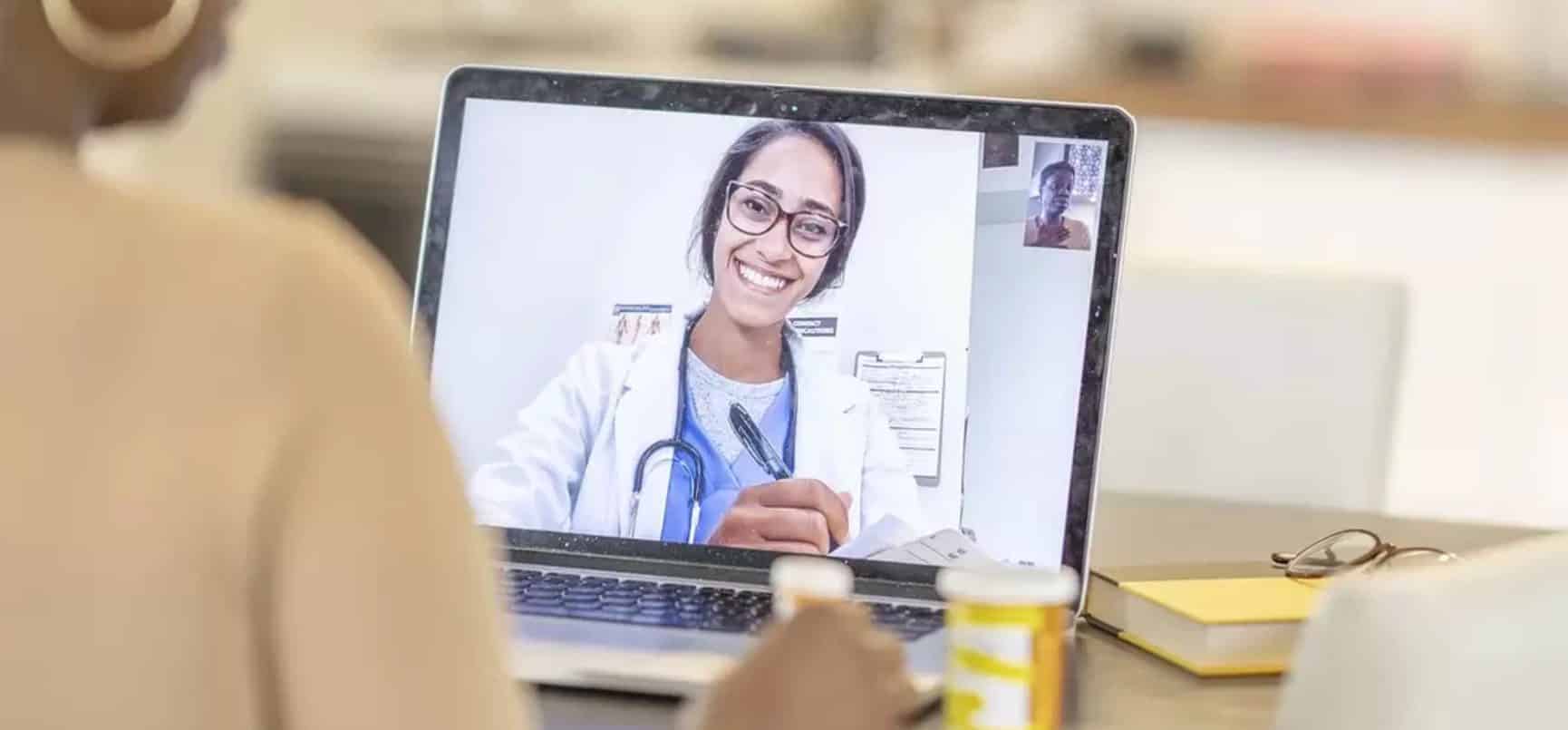
Emerging from the pandemic, telehealth and virtual care also present a new challenge for the Biden administration. As a result of COVID-19, CMS relaxed telehealth regulatory requirements to expand access. Under Biden, Sidley states that telehealth services will likely continue to grow. However, the expansion of telehealth services renews efforts to regulate and scrutinize service providers to ensure adequate access. The 2021 Physician Fee Schedule further expands digital healthcare but also invites stricter enforcement of these billing codes. These provisions extend to data security concerns, too. Sidley recommends that companies prioritize data security and compliance measures.
Law firms like Holland & Knight LLP have also listed expectations of increased price transparency, 340B disputes about pharmaceutical manufacturers and drug prices, and challenges to antitrust and healthcare consolidation. As such issues mount, stringent healthcare and life science regulation present challenges to compliance and mergers.